Consortium Nano4Tarmed is developing 2D nanoplatforms for active drug delivery and diagnosis of osteosarcoma.
Population growth is shrinking, mainly due to the overall fertility rate that dropped from 1.59 in 2016 to 1.53 in 2019 in the European Union.1 An expected decrease in the following years means that the European population is constantly decreasing.
The U.S. Census Bureau expects that the European Union will witness a 14% decrease in the workforce and a 7% decrease in the consumer population by 2030.2 Children’s health care is therefore fundamental to challenge this prediction.
The condition of primary children’s health-care impacts not only the quality of life of the youngest members of our society but also its overall size. Osteosarcoma is one of the most common malignant bone tumours in children and young adults, with more than 900 cases diagnosed each year in the US.
To address this threat, the Nano4Tarmed project aims to develop a new strategy for the treatment of osteosarcoma using a new generation of 2D theranostic nanoplatforms. Current chemotherapy-based treatment usually includes doxorubicin, cisplatin, methotrexate, ifosfamide, and etoposide.4 Notably, the introduction of multimodal treatment improved the overall survival of 65% of treated patients over five years.5 Yet, relapsing or metastatic stages still present fatal outcomes, and side effects of traditional chemotherapy often severely affect the quality of a patient’s life and even shorten the overall survival.
Thus, considerable efforts are still required to personalise new therapeutic strategies directed at reducing cancer recurrence and defining new potential adjuvant targets for inhibiting invasion and metastasis. Combination therapy, based on two or more drugs with various modes of action, potentially represents an efficient strategy for cancer treatment. The expected increase in the efficacy of such therapeutic formulation strongly relates to the ability of the drugs to selectively target diseased tissue while overcoming biological barriers and smartly responding to the diseased environment to release therapeutic agents.
One method with considerable potential is the development of drug delivery systems to minimise risks introduced by conventional treatment strategies. Interestingly, nanomaterials play essential roles in the construction of these drug delivery solutions. Their unique features, including optoelectronic, physical, and toxicological properties, are frequently used to construct devices for multiplex imaging, molecular sensing, photoacoustic imaging, photothermal therapy, and point-of-care medical appliances.
Nanomaterials are also being actively tested in various nanotherapies. Critical scientific challenges recognised in the development of active drug delivery systems are:
- Selectivity for the interaction with cancer cells;
- The ability to bind molecular drugs and release them in the targeted place;
- Biocompatibility of the platform;
- Characterised distribution profiles across the targeted living system; and
- Appropriate knowledge of the elimination mechanism.
About the Nano4tarmed project
The Nano4Tarmed project established an interdisciplinary consortium consisting of the Regional Centre of Advanced Technologies and Materials (RCPTM, part of Palacký University Olomouc), the National Research Council of Italy (CNR), and the Department of Chemistry in Maynooth University (MU, Ireland), which developed a capable scientific cluster through a set of workshops, seminars, and extensive exchange of researchers.
The scientific topics of the cluster aim to develop intelligent nanostructures for targeted drug delivery and signalling. Research activities of the consortium can be divided into three interconnected research blocks: the synthesis of novel anticancer drugs, the development and synthesis of 2D nanoplatforms, and the evaluation of the toxicity and anticancer activity of drug-loaded platforms on cellular models. The first activity, i.e., the synthesis of novel platinum-based anticancer drugs, is performed in Dr Montagner’s the chemical laboratory at the Department of Chemistry in Maynooth University.
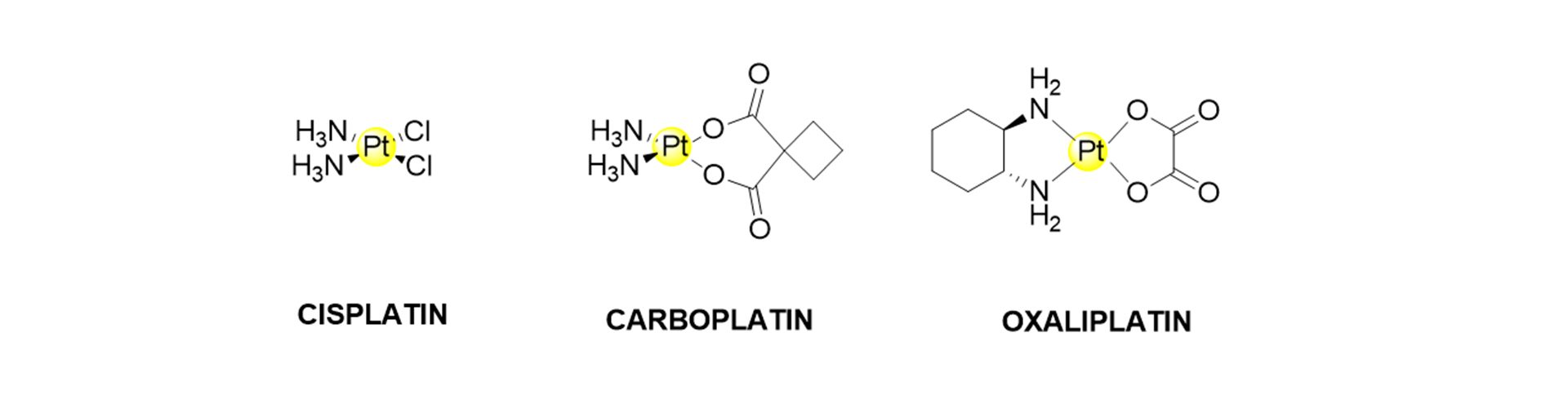
Cisplatin is the most critical chemotherapeutic drug used worldwide to treat different cancers, including osteosarcoma. The structure of cisplatin and its common derivatives are shown in Fig. 1. The main drawbacks associated with its use are the lack of selectivity for cancer cells and its high toxicity. Cisplatin and other approved platinum chemotherapeutic drugs (i.e. carboplatin and oxaliplatin) are nephro and ototoxic. In many cases, tumours develop resistance mechanisms after several chemotherapeutic cycles, and the therapy must be halted.
One strategy under investigation is the use of specific vectors that could deliver platinum-based drugs to the targeted tissue, such as carbohydrates whose receptors are overexpressed in cancer cell membranes. Within the Nano4TarMed project, Montagner’s group is developing new platinum-based drugs that could be anchored to the nanoplatform developed by the Ranc lab at Palacký University to enhance tumour selectivity and diminish the toxic side effects.
The second activity, namely the synthesis and development of 2D nanoplatforms, is performed at Palacký University in Olomouc by Dr Ranc. The developed 2D nanoplatforms are currently based on using graphene oxide as the first component, which is planned to be replaced in the following phases by the new generation of more potent carbon derivatives, including graphene acid.
Graphene oxide is functionalised by highly branched polymers, which increases the system biocompatibility and introduces important functional groups ready for the anchoring of drugs, sensors, and linkers. The topography, morphology, chemical composition, and stability are afterwards studied and described using techniques of molecular and atomic spectroscopy, electron microscopy, and atomic force microscopy.
The goal is to provide an intelligent drug delivery system and its in-detail characterisation. In the following steps, the developed nanoplatforms are functionalised using a combination of anticancer drugs provided by Dr Montagner’s lab and selected convent drugs.
The stability of such loaded drug delivery systems is tested under laboratory conditions at various pH levels and temperatures. Final drug-loaded nanoplatforms then enter the third project research phase: testing of the anticancer activity. The testing of the anticancer activity of the developed drug delivery systems and new platinum-based drugs is provided by the Cell-Material BioLAB of the CNR, coordinated by Dr. Panseri. This laboratory is focused on studying cell-material interaction using cellular and molecular biology techniques to evaluate the biocompatibility and bifunctionality of novel drugs and biomaterials for nanomedicine.
The aim of N4T is to understand how the novel nanostructured materials, coupled with Pt-based drug synthesised by the other two partners, could work as an intelligent therapy that allows us to use the drug in lower doses than the current average. The development could hit tumour cells selectively and avoid treatments affecting those cells that are not affected, which is standard practice in chemotherapy.
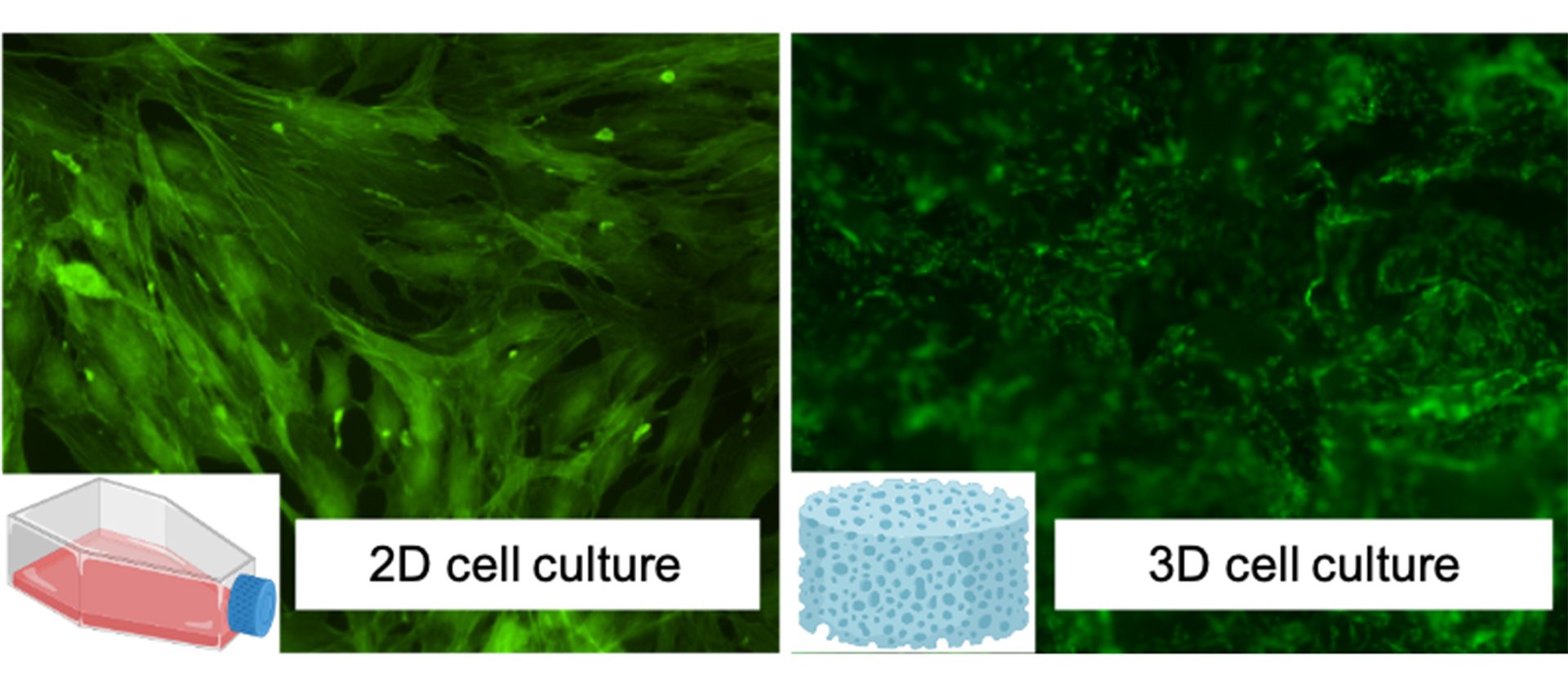
The project applies the experimental activity to osteosarcomas, a particular type of bone cancer that affects adolescents, and two other main categories of tumours with high incidence and morbidity worldwide (breast and brain). After an initial screening using standard in vitro tests where cytotoxicity and bioactivity of new systems have been evaluated, CNR is now moving to more complex cell culture models. Bidimensional (2D) culture systems still represent the gold standard models for in vitro research as simple, cost-effective, robust, and an excellent high-throughput practice option for cell-based biology studies.
Unfortunately, these systems are not able to recapitulate in vivo three-dimensional (3D) environments, resulting in a poor in vitro–in vivo translation, as shown in Fig. 2. In addition, differences in intra-species limited the use of animal data for predicting human responses, increasing in vivo preclinical failures and ethical concerns. Dealing with these challenges, in vitro 3D technological approaches were recently bioengineered as promising platforms able to closely capture the complexity of in vivo normal/pathological tissues.
In the Nano4Tarmed project, CNR, thanks to biomaterials (i.e., collagen-based scaffolds), is carrying out innovative three-dimensional tests to test the effectiveness of drug delivery. These studies provide:
- Optimal and promising platforms to closely capture in vivo microenvironment in a laboratory setting;
- Seal the gap between the 2D culture systems and animal models;
- Improve test outcomes; and
- Decrease the use of animals for in vivo studies.
Results of the nanoplatform testing
The fully established consortium has produced the pilot version of the drug delivery nanoplatform for transporting platinum-based complexes and has successfully tested its performance on three cancerous cells, including osteosarcoma, breast cancer, and gliomas. The large surface area makes carbon-based nanomaterials excellent drug carrier candidates.
The pilot study showed that a GO-based nano platform PEGylated with 8-arm polyethene glycol improves the functionalisation with a platinum drug based on a cisplatin scaffold. The obtained data demonstrated that the GO@PEG carrier allows less Pt-based drug while reaching an excellent cellular proliferation inhibition in osteosarcoma. The altered cellular internalisation was also observed in glioblastoma, but due to different cell metabolism, the Pt drug bioactivity, once inside the cells, was less pronounced.
The proposed GO@PEG nanoplatform is also promising in inhibiting migration, especially in highly invasive breast carcinoma (i.e., MDA-MB-231 cell line), neutralising the metastatic process. We thus demonstrated that the developed GO@PEG nano platform represents a promising strategy in nanomedicine for cancer treatment due to its drug-loading capacity, which can be specifically tailored to target different cancers.6
References:
- Cafaro, Philip, and Patrícia Dérer. “Policy-based population projections for the European Union: a complementary approach.” Comparative Population Studies 44 (2019).
- Mason, Andrew, Ronald Lee, and NTA Network. “Six ways population change will affect the global economy.” Population and development review 48.1 (2022): 51-73.
- https://www.boneandjointburden.org/2014-report/viiiab14/economic-cost-malignant-bone-tumors
- Oualla, Karima, et al. “Novel therapeutic strategies in the treatment of triple-negative breast cancer.” Therapeutic advances in medical oncology 9.7 (2017): 493-511.
- Kansara, Maya, et al. “Translational biology of osteosarcoma.” Nature Reviews Cancer 14.11 (2014): 722-735.
- Giusto, Elena, et al. “Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy.” Nanomaterials 12.14 (2022): 2372.